AEM Water Electrolysis: How it Works
As the world’s leading manufacturer of AEM electrolysers, we at Enapter feel responsible for sharing everything you need to know about how AEM electrolysis works
Green hydrogen produced from water using renewable energy is recognised as the most promising energy carrier we have for fully replacing fossils fuels in many sectors. A handful of different technologies used in producing green hydrogen have been developed over the last century, but a highly important piece of the puzzle was missing for a long time. Enapter has completed this puzzle with its anion exchange membrane (AEM) technology for water electrolysis.
But before we look at the AEM, we need to answer the question: Why can we store energy in hydrogen?
Water electrolysis is the reaction of splitting water molecules into hydrogen and oxygen. It is an endothermic process, which means it can absorb and store energy in the form of chemical bonds in hydrogen. The reverse reaction of hydrogen and oxygen forming water molecules, on the other hand, is an exothermic reaction that releases energy to its surroundings. Using the cycle of endothermal water electrolysis and subsequent exothermal water forming reactions, we can store renewable energy in green hydrogen – without fossil fuels and carbon dioxide emissions.
How AEM stacks up
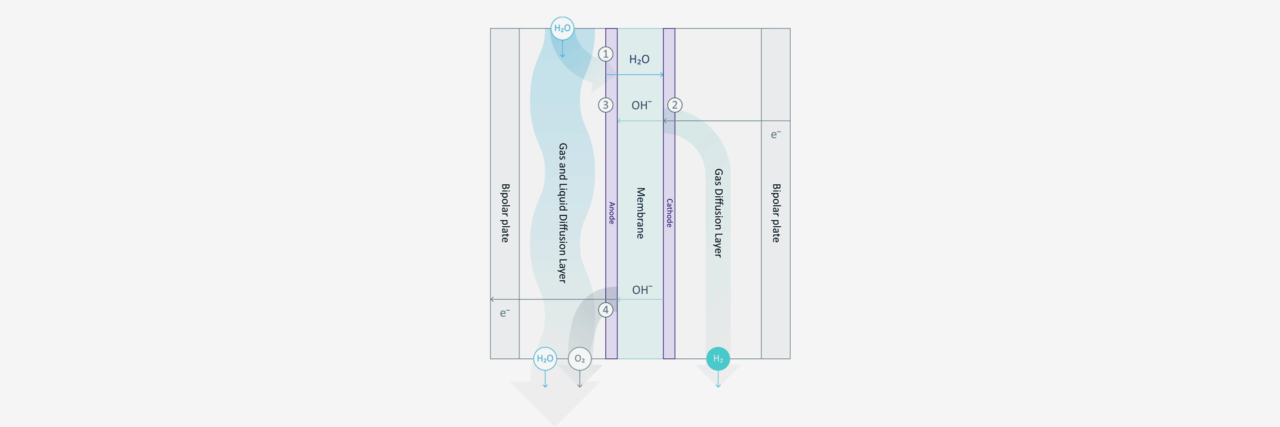
Before diving into the mechanisms of AEM electrolysis, we need to first understand the most essential component in an AEM electrolyser: the AEM stack, where the water splitting reaction happens. As shown in the diagram, the single cell is separated into two half-cells by the anion exchange membrane. Each half-cell consists of an electrode, a gas diffusion layer (GDL), and a bipolar plate (BPP). Multiple single cells are connected by the bipolar plate to form the AEM stack.
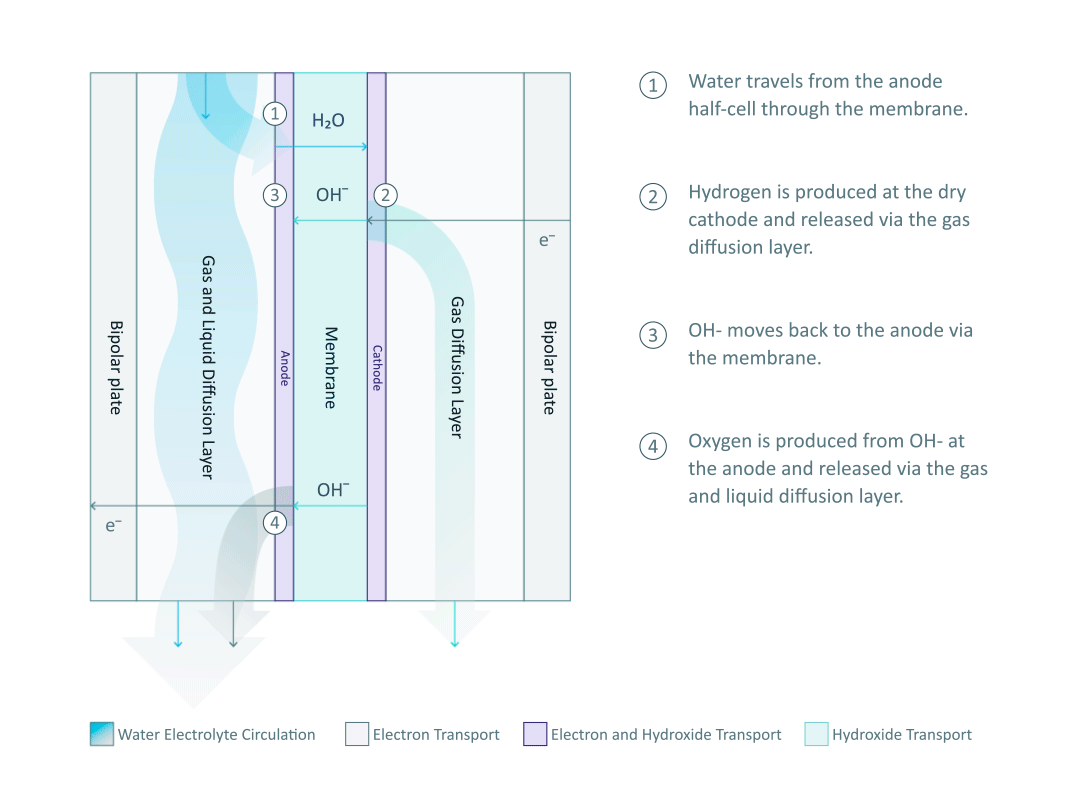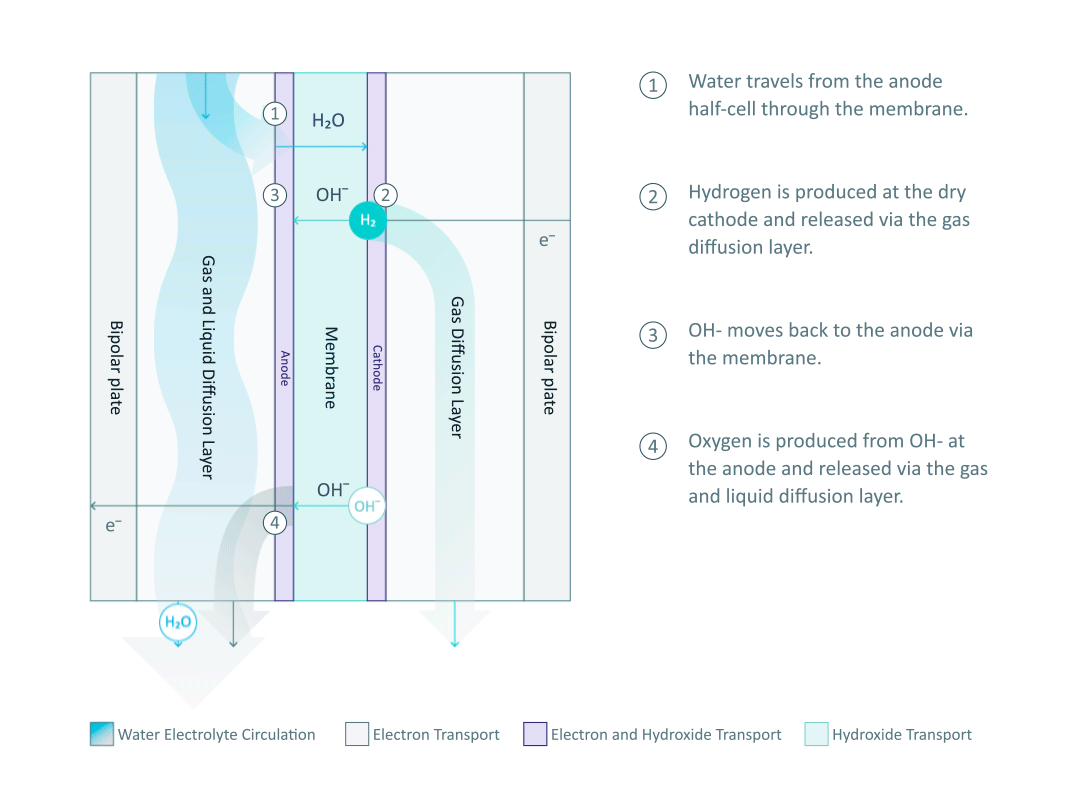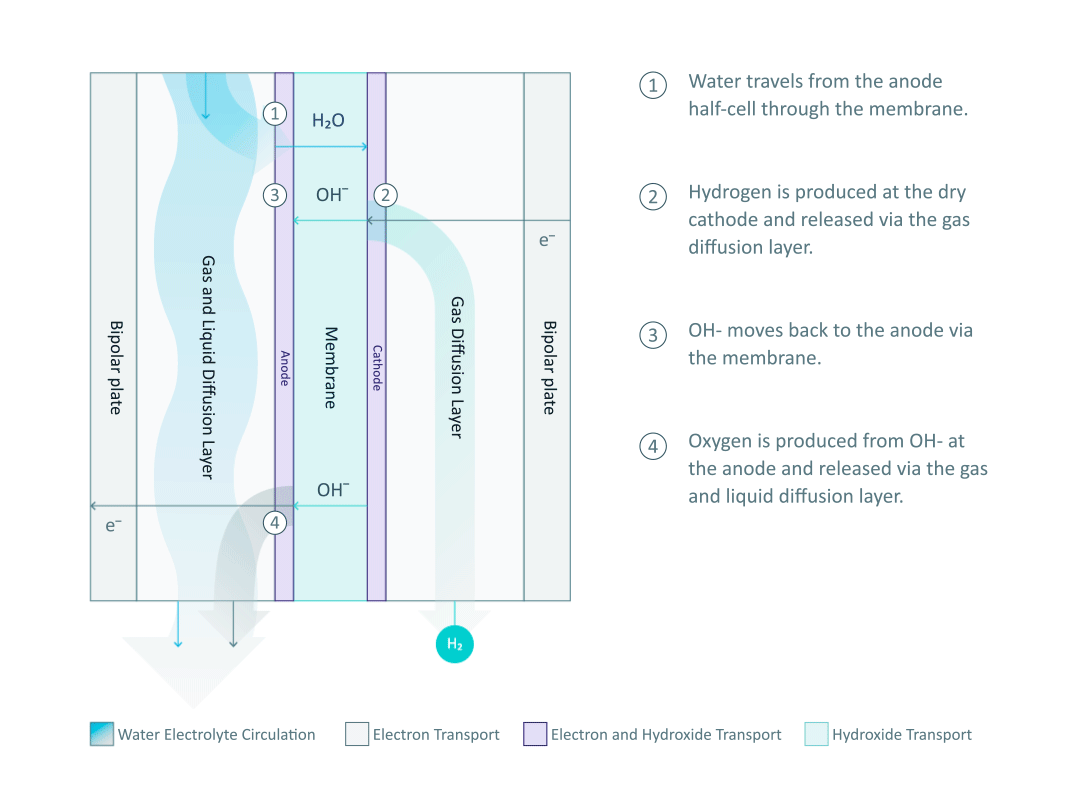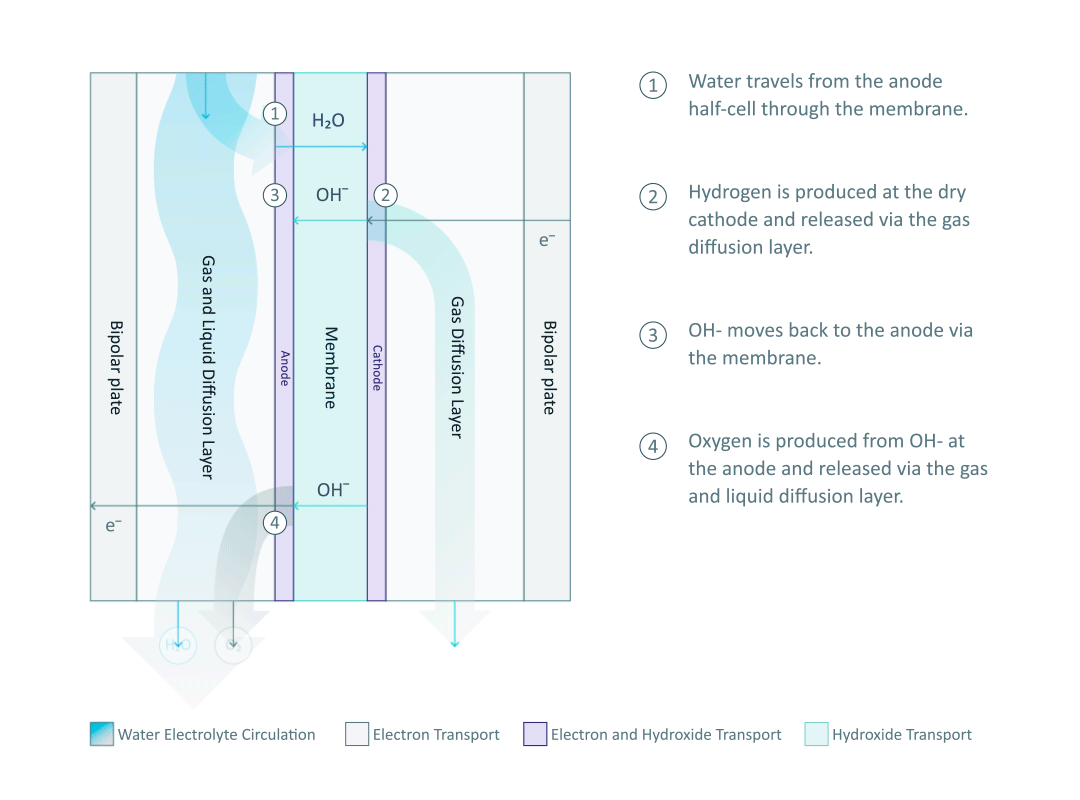
The half-cell arrangement in an AEM electrolyser, unlike in a traditional alkaline (TA) electrolyser, allows the hydrogen and oxygen to be produced under pressure of 35 bar and 1 bar, respectively. The pressure difference between the half-cells can prevent the produced oxygen from crossing over to the high-pressure half-cell, thus ensuring that the hydrogen has very high purity (99.9 %).
Splitting the H from the H2O
The water electrolyte, containing just 1% potassium hydroxide (KOH), only circulates in the anode half-cell and wets the membrane, while the cathode side remains dry. Therefore, the hydrogen produced from the cathode half-cell has a low moisture content, and it is important to note that no KOH can be found in the cathode half-cell. The water molecules travel through the membrane and are reduced at the cathode to produce hydrogen. The power supply from the external circuit is used to create an electrical potential difference at the interface of the electrolyte and electrode. The potential difference then drives the hydrogen evolution reaction (HER) by means of electron (e-) transfer: 4H2O + 4e- → 4OH- + 2H2.
The produced hydrogen is then released through the GDL to the output pipeline. Appropriate HER catalysts at the cathode facilitate the process by lowering the energy barrier of the reaction.
pH and the oxygen evolution
In the mild alkaline environment of the AEM electrolyser, the remaining hydroxide ion (OH-) from the HER will return to the anode half-cell via the membrane. The exchanged OH- is an anion, which gives the AEM its name. In a proton exchange membrane (PEM) electrolyser, the proton (H+) is transported through the PEM in a highly acidic environment.
Therefore, the PEM electrolyser requires platinum group metals (PGM) as catalysts and expensive titanium bipolar plates to survive the highly corrosive acidic environment, while non-PGM catalysts and steel bipolar plates are sufficient for effective hydrogen production in the AEM electrolyser. the diluted KOH solution in an AEM electrolyser is much safer to handle than the electrolyte with a pH of 14 in a TA electrolyser.
After the OH- is transported back to the anode side of an AEM electrolyser, it is consumed by the oxygen evolution reaction (OER): 4OH- → 2H2O + O2 + 4e-. For every two units of hydrogen, one unit of oxygen is generated by transferring four units of electrons. Hence, the OH- concentration in the electrolyte can remain constant through constantly supplying water without adding more KOH. The OER is driven by the potential difference at the catalytic sites on the anode and the produced oxygen is removed from the anode half-cell via GDL along with the electrolyte circulation.
Using AEM water electrolysis, Enapter’s modular electrolysers can produce 500 NL of green hydrogen per hour, with a purity of 99.9 % (99.999 % after drying) at 35 bar pressure from 0.4 L of water and 2.4 kWh of renewable energy. We think these results speak for themselves – but we warmly invite you to reach out to our team if you still have questions about how creating green hydrogen through AEM electrolysis can work for you.
by Jingwen Wang
Learn more about the benefits of our AEM electrolysers and how we are advancing our R&D projects with a powerful Scanning Electron Microscope.